How does Good Manufacturing Practices and ERP work together?
Within the Food and Beverage industry, a common voiced concern is audits and regulatory compliance, and across the industry there are many standards, such as Safe Quality Food (SQF), British Retail Consortium (BRC) and others which are HACCP based food safety and quality management systems
Good Manufacturing Practices (GMP) and Hazard Analysis and Critical Control Points (HACCP) are both systems intended to ensure the safe production of food. GMP is the “first step” to food safety; a series of principles to be fulfilled to ensure that products meet food safety requirements. GMPs are the fundamental components of HACCP, which is a systematic approach to production that is designed to prevent and control hazards before food safety is compromised.
A GMP plan is a combination of management and manufacturing practices, procedures and policies which are well documented and designed to ensure food products are consistently processed to comply with safety standards.
How does ERP fit into this? well the marriage between GMP and ERP are these documented procedures and policies where the business and corresponding ERP functions and process flows are cross referenced.
Depending on the type and nature of the product being produced, and defined GMP and HACCP protocols, quality control testing may occur at different points of the production process, and quarantine controls for batches in the post production phase. The illustration below is such an example:
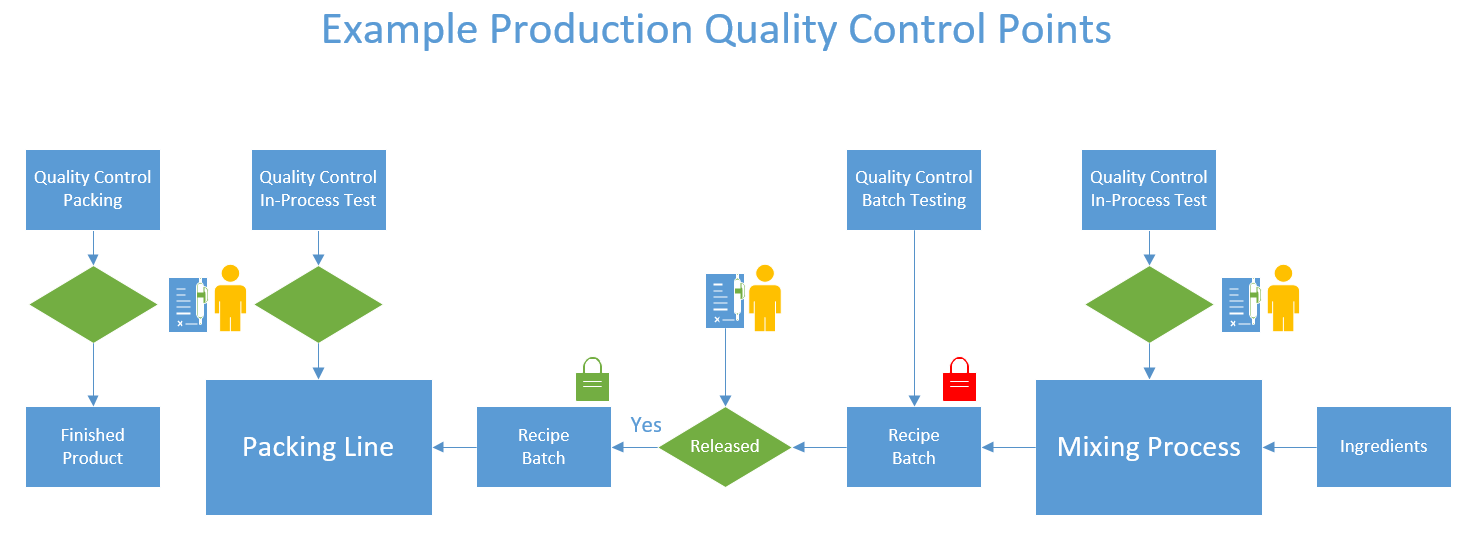 To support such GMP’s , how does ProcessForce manage this process ? and what is the configuration required to achieve this ? below are some screen examples.
To support such GMP’s , how does ProcessForce manage this process ? and what is the configuration required to achieve this ? below are some screen examples.
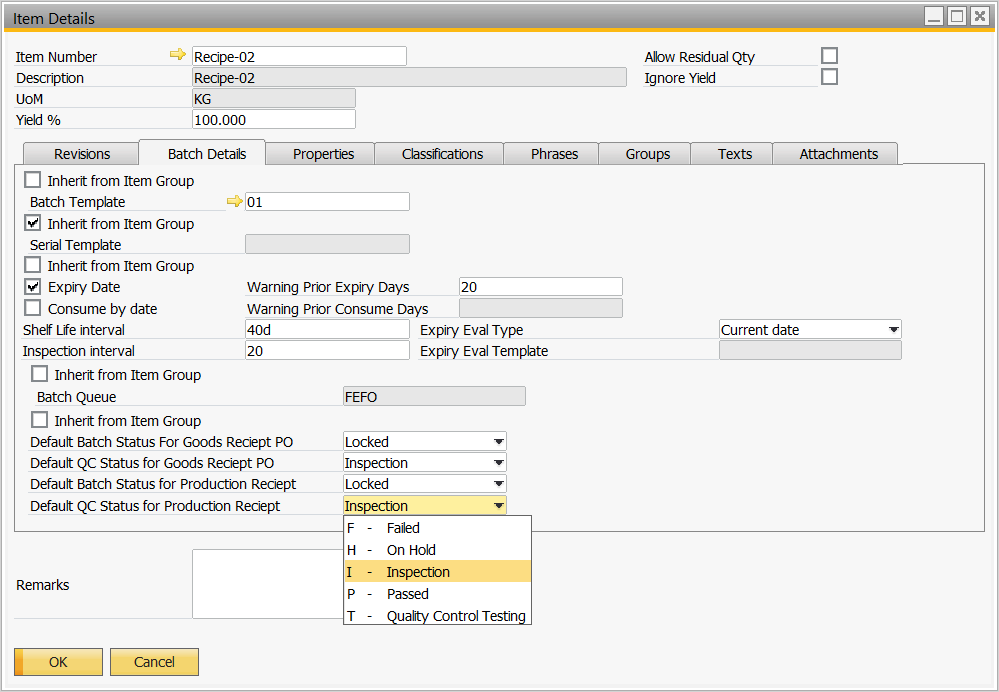
Set the Item so when the batch is produced it will be ‘Locked’ and status ‘Inspection’
Batch Release is based on performing a Quality Control test directly from the batch
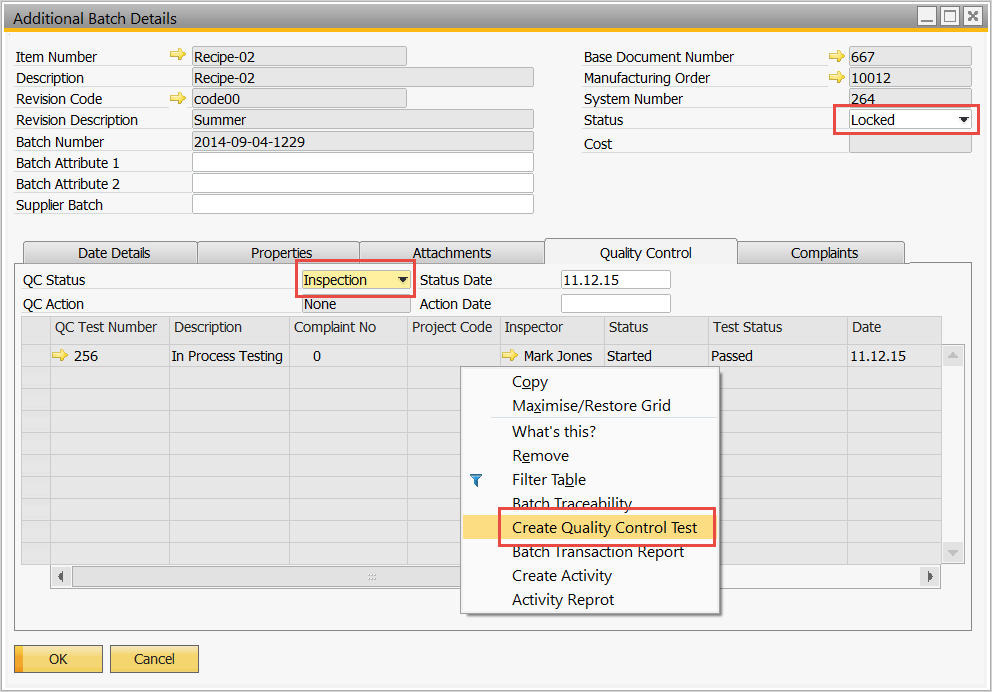
Perform the Quality Control Test and validate the results
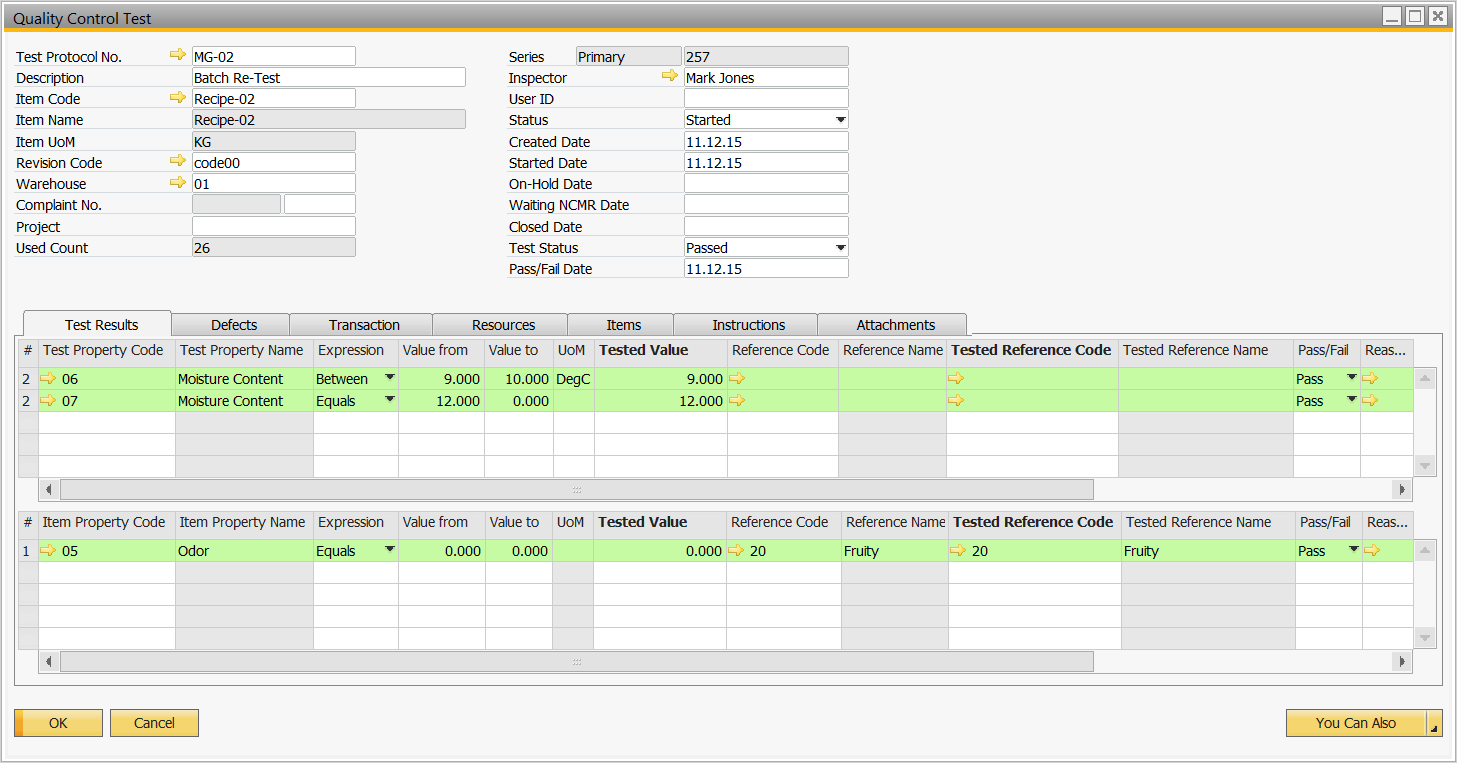
Batch is passed and ready to be consumed for the next phase of production